Introduction:
Fluid Mechanics is that section of applied mechanics, concerned with the statics and dynamics of liquids and gases.
Fluid Statics: Which treats fluids in the equilibrium state of no shear stress
Fluid Mechanics: Which treats when portions of fluid are in motion relative to other parts.
Fluids
A fluid is a substance which deforms continuously under the action of shearing forces, however small they may be.Conversely, it follows that:
If a fluid is at rest, there can be no shearing forces acting and, therefore, all forces in the fluid must be perpendicular to the planes upon which they act.
Shear stress in a moving fluid
Although there can be no shear stress in a fluid at rest, shear stresses are developed when the fluid is in motion, if the particles of the fluid move relative to each other so that they have different velocities, causing the original shape of the fluid to become distorted. If, on the other hand, the velocity of the fluid is same at every point, no shear stresses will be produced, since the fluid particles are at rest relative to each other.
Types Of Fluids:
Newtonian fluids:
Fluids which obey the Newton's law of viscosity are called as Newtonian fluids. Newton's law of viscosity is given by
t = mdv/dy
where t = shear stress
m = viscosity of fluid
dv/dy = shear rate, rate of strain or velocity gradient
All gases and most liquids which have simpler molecular formula and low molecular weight such as water, benzene, ethyl alcohol, CCl4, hexane and most solutions of simple molecules are Newtonian fluids.
Non-Newtonian fluids:
Fluids which do not obey the Newton's law of viscosity are called as non-Newtonian fluids.
Generally non-Newtonian fluids are complex mixtures: slurries, pastes, gels, polymer solutions etc.,
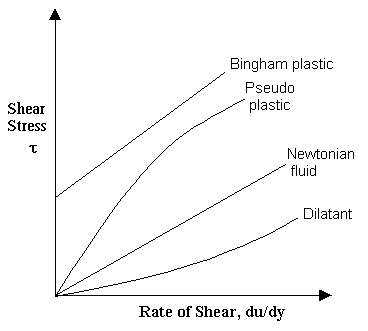
Various non-Newtonian Behaviors:
Time-Independent behaviors:
Properties are independent of time under shear.
Bingham-plastic: Resist a small shear stress but flow easily under larger shear stresses. e.g. tooth-paste, jellies, and some slurries.
Pseudo-plastic: Most non-Newtonian fluids fall into this group. Viscosity decreases with increasing velocity gradient. e.g. polymer solutions, blood. Pseudoplastic fluids are also called as Shear thinning fluids. At low shear rates(du/dy) the shear thinning fluid is more viscous than the Newtonian fluid, and at high shear rates it is less viscous.
Dilatant fluids: Viscosity increases with increasing velocity gradient. They are uncommon, but suspensions of starch and sand behave in this way. Dilatant fluids are also called as shear thickening fluids.
Time dependent behaviors:
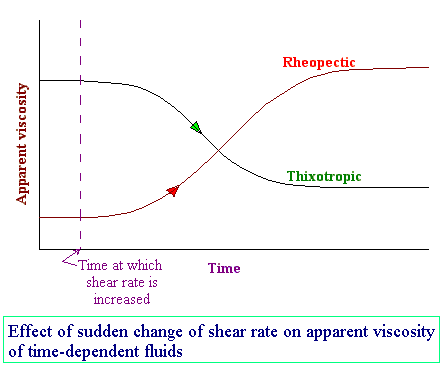
Those which are dependent upon duration of shear.
Thixotropic fluids: for which the dynamic viscosity decreases with the time for which shearing forces are applied. e.g. thixotropic jelly paints.
Rheopectic fluids: Dynamic viscosity increases with the time for which shearing forces are applied. e.g. gypsum suspension in water.
Visco-elastic fluids: Some fluids have elastic properties, which allow them to spring back when a shear force is released. e.g. egg white.
Surface Tension-
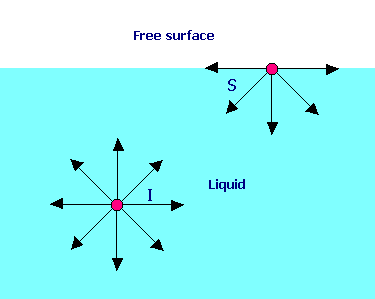
A molecule I in the interior of a liquid is under attractive forces in all directions and the vector sum of these forces is zero. But a molecule S at the surface of a liquid is acted by a net inward cohesive force that is perpendicular to the surface. Hence it requires work to move molecules to the surface against this opposing force, and surface molecules have more energy than interior ones.
The surface tension (s sigma) of a liquid is the work that must be done to bring enough molecules from inside the liquid to the surface to form one new unit area of that surface (J/m2 = N/m). Historically surface tensions have been reported in handbooks in dynes per centimeter (1 dyn/cm = 0.001 N/m).
Surface tension is the tendency of the surface of a liquid to behave like a stretched elastic membrane. There is a natural tendency for liquids to minimize their surface area. For this reason, drops of liquid tend to take a spherical shape in order to minimize surface area. For such a small droplet, surface tension will cause an increase of internal pressure p in order to balance the surface force.
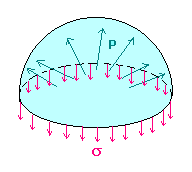 We will find the amount D (Dp = p - poutside) by which the pressure inside a liquid droplet of radius r, exceeds the pressure of the surrounding vapor/air by making force balances on a hemispherical drop. Observe that the internal pressure p is trying to blow apart the two hemispheres, whereas the surface tension s is trying to pull them together. Therefore, Dp pr2 = 2prs
We will find the amount D (Dp = p - poutside) by which the pressure inside a liquid droplet of radius r, exceeds the pressure of the surrounding vapor/air by making force balances on a hemispherical drop. Observe that the internal pressure p is trying to blow apart the two hemispheres, whereas the surface tension s is trying to pull them together. Therefore, Dp pr2 = 2prsi.e. Dp = 2s/r
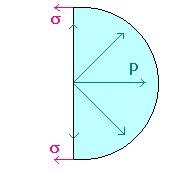 Similar force balances can be made for cylindrical liquid jet.
Similar force balances can be made for cylindrical liquid jet.Dp 2r= 2s
i.e. Dp = s/r
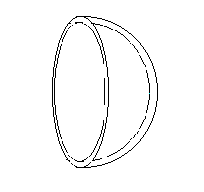 Similar treatment can be made for a soap bubble which is having two free surfaces. Dp pr2 = 2 x 2prs
Similar treatment can be made for a soap bubble which is having two free surfaces. Dp pr2 = 2 x 2prsi.e. Dp = 4s/r
Surface tension generally appears only in situations involving either free surfaces (liquid/gas or liquid/solid boundaries) or interfaces (liquid/liquid boundaries); in the latter case, it is usually called the interfacial tension.
Rise or fall of a liquid in a capillary tube is caused by surface tension and depends on the relative magnitude of cohesion of the liquid and the adhesion of the liquid to the walls of the containing vessel.
Liquids rise in tubes if they wet (adhesion > cohesion) and fall in tubes that do not wet (cohesion > adhesion).
Capllirarty
Wetting and contact angle
Fluids wet some solids and do not others.
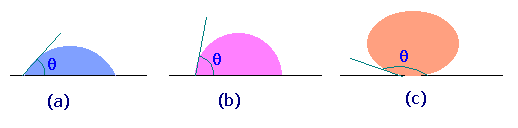 The figure shows some of the possible wetting behaviors of a drop of liquid placed on a horizontal, solid surface (the remainder of the surface is covered with air, so two fluids are present).
The figure shows some of the possible wetting behaviors of a drop of liquid placed on a horizontal, solid surface (the remainder of the surface is covered with air, so two fluids are present).
Fig.(a) represents the case of a liquid which wets a solid surface well, e.g. water on a very clean copper. The angle q shown is the angle between the edge of the liquid surface and the solid surface, measured inside the liquid. This angle is called the contact angle and is a measure of the quality of wetting. For perfectly wetting, in which the liquid spreads as a thin film over the surface of the solid, q is zero.
Fig.(c) represents the case of no wetting. If there were exactly zero wetting, q would be 180o. However, the gravity force on the drop flattens the drop, so that 180o angle is never observed. This might represent water on teflon or mercury on clean glass.
We normally say that a liquid wets a surface if q is less than 90o and does not wet if q is more than 90o. Values of q less than 20o are considered strong wetting, and values of q greater than 140o are strong nonwetting.
Capillarity is important (in fluid measurments) when using tubes smaller than about 10 mm in diameter.
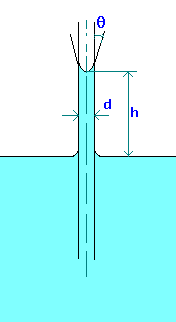
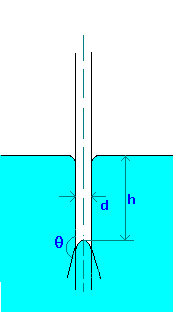 Capillary rise (or depression) in a tube can be calculated by making force balances. The forces acting are force due to surface tension and gravity.
Capillary rise (or depression) in a tube can be calculated by making force balances. The forces acting are force due to surface tension and gravity.The force due to surface tesnion,
Fs = pdscos(q), where q is the wetting angle or contact angle. If tube (made of glass) is clean q is zero for water and about 140o for Mercury.
This is opposed by the gravity force on the column of fluid, which is equal to the height of the liquid which is above (or below) the free surface and which equals
Fg = (p/4)d2hgr,
where r is the density of liquid.
Equating these forces and solving for Capillary rise (or depression), we find
h = 4scos(q)/(rgd)
Fluid Statics: Study of fluid at rest.
Pascal Law:
h = 4scos(q)/(rgd)
Fluid Statics: Study of fluid at rest.
Pascal Law:
The basic property of a static fluid is pressure. Pressure is familiar as a surface force exerted by a fluid against the walls of its container. Pressure also exists at every point within a volume of fluid. For a static fluid, as shown by the following analysis, pressure turns to be independent direction.

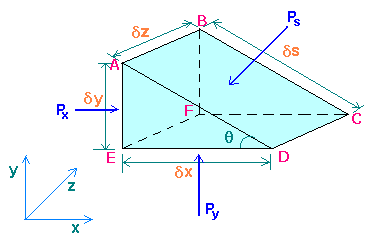
By considering the equilibrium of a small fluid element in the form of a triangular prism ABCDEF surrounding a point in the fluid, a relationship can be established between the pressures Px in the x direction, Py in the y direction, and Ps normal to any plane inclined at any angle q to the horizontal at this point.
Px is acting at right angle to ABEF, and Py at right angle to CDEF, similarly Ps at right angle to ABCD.
Since there can be no shearing forces for a fluid at rest, and there will be no accelerating forces, the sum of the forces in any direction must therefore, be zero. The forces acting are due to the pressures on the surrounding and the gravity force.
Force due to Px = Px x Area ABEF = Pxdydz
Horizontal component of force due to Ps = - (Ps x Area ABCD) sin(q) = - Psdsdz dy/ds = -Psdydz
As Py has no component in the x direction, the element will be in equilibrium, if
Pxdydz + (-Psdydz) = 0
i.e. Px = Ps
Similarly in the y direction, force due to Py = Pydxdz
Component of force due to Ps = - (Ps x Area ABCD) cos(q) = - Psdsdz dx/ds = - Psdxdz
Force due to weight of element = - mg = - rVg = - r (dxdydz/2) g
Since dx, dy, and dz are very small quantities, dxdydz is negligible in comparison with other two vertical force terms, and the equation reduces to,
Py = Ps
Therefore, Px = Py = Ps
i.e. pressure at a point is same in all directions. This is Pascal's law. This applies to fluid at rest.
Fine powdery solids resemble fluids in many respects but differs considerably in others. For one thing, a static mass of particulate solids, can support shear stresses of considerable magnitude and the pressure is not the same in all directions.
Variation of pressure with elevation

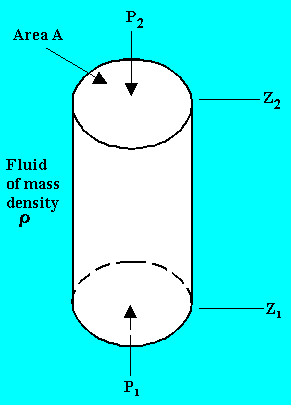
Consider a hypothetical differential cylindrical element of fluid of cross sectional area A and height (z2 - z1).
Upward force due to pressure P1 on the element = P1A
Downward force due to pressure P2 on the element = P2A
Force due to weight of the element = mg = rA(z2 - z1)g
Equating the upward and downward forces,
P1A = P2A + rA(z2 - z1)g
P2 - P1 = - rg(z2 - z1)
Thus in any fluid under gravitational acceleration, pressure decreases, with increasing height z in the upward direction.
Equality of pressure at the same level in a static fluid:

Equating the horizontal forces, P1A = P2A (i.e. some of the horizontal forces must be zero)
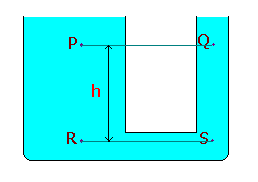
Equality of pressure at the same level in a continuous body of fluid:

Pressures at the same level will be equal in a continuous body of fluid, even though there is no direct horizontal path between P and Q provided that P and Q are in the same continuous body of fluid.
We know that, PR = PS
PR = PP + rgh à 1
PS = PQ + rgh à 2
From equn.1 and 2, PP = PQ
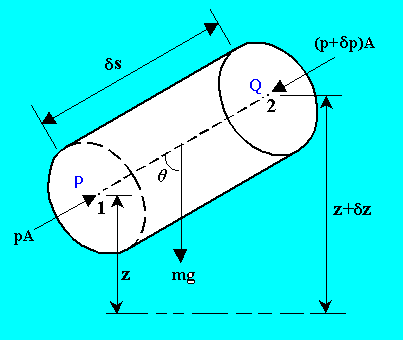
General equation for the variation of pressure due to gravity from point to point in a static fluid:

Resolving the forces along the axis PQ,
pA - (p + dp)A - rgAds cos(q) = 0
dp = - rgds cos(q)
or in differential form,
dp/ds = - rgcos(q)
In the vertical z direction, q = 0.
Therefore,
dp/dz = -rg
This equation predicts a pressure decrease in the vertically upwards direction at a rate proportional to the local density.
Absolute Pressure, Gauge Pressure and Vacuum Pressure
In a region such as outer space, which is virtually void of gases, the pressure is essentially zero. Such a condition can be approached very nearly in a laboratory when a vacuum pump is used to evacuate a bottle. The pressure in a vacuum is called absolute zero, and all pressures referenced with respect to this zero pressure are termed absolute pressures.
Many pressure-measuring devices measure not absolute pressure but only difference in pressure. For example, a Bourdon-tube gage indicates only the difference between the pressure in the fluid to which it is tapped and the pressure in the atmosphere. In this case, then, the reference pressure is actually the atmospheric pressure. This type of pressure reading is called gauge pressure.
For example, if a pressure of 50 kPa is measured with a gage referenced to the atmosphere and the atmospheric pressure is 100 kPa, then the pressure can be expressed as either
p = 50 kPa gage or p = 150 kPa absolute.
p = 50 kPa gage or p = 150 kPa absolute.
Whenever atmospheric pressure is used as a reference, the possibility exists that the pressure thus measured can be either positive or negative. Negative gauge pressure are also termed as vacuum pressures.
Hence, if a gauge tapped into a tank indicates a vacuum pressure of 31 kPa, this can also be stated as 70 kPa absolute, or -31 kPa gage, assuming that the atmospheric pressure is 101 kPa absolute.
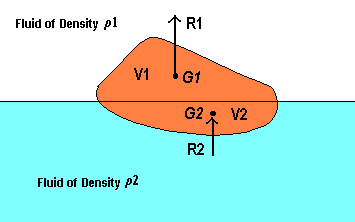
Buoyancy
Upthrust on body = weight of fluid displaced by the body
This is known as Archimedes principle.
This is known as Archimedes principle.

If the body is immersed so that part of its volume V1 is immersed in a fluid of density r1 and the rest of its volume V2 in another immiscible fluid of mass density r2,
Upthrust on upper part, R1 = r1gV1
acting through G1, the centroid of V1,
Upthrust on lower part,R2 = r2gV2
acting through G2, the centroid of V2,
Total upthrust = r1gV1 + r2gV2.
The positions of G1 and G2 are not necessarily on the same vertical line, and the centre of buoyancy of the whole body is, therefore, not bound to pass through the centroid of the whole body.
Upthrust on upper part, R1 = r1gV1
acting through G1, the centroid of V1,
Upthrust on lower part,R2 = r2gV2
acting through G2, the centroid of V2,
Total upthrust = r1gV1 + r2gV2.
The positions of G1 and G2 are not necessarily on the same vertical line, and the centre of buoyancy of the whole body is, therefore, not bound to pass through the centroid of the whole body.
